Gene Therapy Formulation Endotoxin Testing
Inquiry
Endotoxin testing is a crucial quality control step in the development of gene therapy formulations. The presence of endotoxin may cause an immune response in the body, leading to severe adverse reactions. Therefore, ensuring that endotoxin levels in gene therapy products are below a safe threshold is essential. Based on our advanced technology platform and experienced technical team of experts, CD Formulation provides researchers with comprehensive technical support for quality control of gene therapy formulations, particularly for endotoxin testing of gene therapy formulations.
The Importance of Gene Therapy Formulation Endotoxin Testing
- Ensure product safety. Endotoxin can cause severe pathological reactions such as fever and shock even in trace amounts. Therefore, detecting and controlling endotoxin levels in gene therapy formulations is essential to ensure product safety.
- Compliance with regulatory requirements. Testing gene therapy products for endotoxin contamination prior to testing in humans is a necessary step in regulatory requirements.
- Improve product quality. Endotoxin testing and removal improves the purity and safety of gene therapy formulations, thereby enhancing overall product quality.
Explore Our Gene Therapy Formulation Endotoxin Testing
Gel method
This is a qualitative or semi-quantitative detection method, through the principle of horseshoe crab reagent and endotoxin producing agglutination reaction for limit detection or semi-quantitative detection of endotoxin.
Turbidimetric method
Determination of endotoxin content by detecting changes in turbidity during the reaction of limulus amebocyte lysate (LAL) with endotoxin.
Chromogenic substrate method
Determination of endotoxin content by using the amount of chromophore released from a specific substrate by the coagulase produced during the reaction between LAL and endotoxin.
Colorimetric method
Determination of endotoxin by measuring the absorbance or by the reaction of the released p-nitroaniline with an azo reagent to form a specific complex.
The Procedure for Endotoxin Testing in Gene Therapy
Limulus amebocyte lysate (LAL) and recombinant C-factor assays are two commonly used endotoxin assays that are important in the development of gene therapy formulations. When choosing an assay, the decision needs to be based on specific sample characteristics and laboratory conditions. We briefly describe here the steps we take when utilizing these two methods for endotoxin detection.
|
Limulus amebocyte lysate (LAL) |
Recombinant C-factor method |
- Preparation of samples and reagents. Prepare the sample to be tested and the endotoxin standard solution and dilute as necessary.
- Add the sample. Add the sample and standard solution to the pre-warmed 96-well plate.
- Add LAL reagent. Add LAL to each well and mix with gentle shaking.
- Incubation. Incubate the 96-well plate at 37°C for a certain period of time.
- Add colorimetric substrate. Add colorimetric substrate to each well and continue incubation.
- Add termination solution. Terminate the reaction by adding the termination solution.
- Read absorbance. Read the absorbance and calculate the concentration of endotoxin in the sample from the standard curve.
|
- Preparation of samples and reagents. Prepare the sample to be tested and the endotoxin working standard and dilute as necessary.
- Add samples to the assay plate. Add samples and standards to a 96-well plate.
- Prepare substrate reagents. Prepare substrate reagents according to our lab instructions.
- Add substrate reagent and fluorescence detection. Add substrate reagent to each well and incubate at 37°C.
- Data analysis. Fluorescence values are measured using a fluorometric enzyme marker and the endotoxin concentration in the sample is calculated from the standard curve.
|
Our Technologies for Gene Therapy Formulation Endotoxin Testing
| Platforms & Technologies |
Content Description |
| Enzyme-linked immunosorbent assay (ELISA) |
This method is based on an antigen-antibody reaction and detects endotoxin through an enzyme-catalyzed colorimetric reaction with high specificity. |
| Monocyte activation reaction assay (MAT) |
This method utilizes monocytes or monocyte cell lines to mimic the human body's response to endotoxin, and determines the endotoxin content of a test article by measuring the amount of pro-inflammatory cytokines released. |
| Biosensor assay |
This is an emerging endotoxin detection technology that utilizes the high sensitivity and rapid response characteristics of biosensors for rapid detection of endotoxin. |
Highlights of Our Gene Therapy Formulation Endotoxin Testing
- We have a professional research and analysis team and top-notch analytical equipment.
- We can provide a simple and fast quantitative endotoxin detection testing program according to customer requirements.
- We offer flexible solutions for endotoxin detection systems that simplify endotoxin testing from the manufacturing process to the final product batch release.
- We specialize in endotoxin testing and have many years of experience with international advanced testing levels.
Published Data
Technology: Endotoxin removal technology
Journal: Mol Ther Methods Clin Dev
IF: 4.6
Published: 2019
Endotoxin contamination in protein samples and recombinant adeno-associated virus (rAAV) stocks can cause severe reactions and is challenging to remove without losing viral titer. This issue is intensified by the variety of rAAV serotypes and capsid variants, which interact differently with endotoxins. This article presents a universal protocol for purifying rAAV stocks regardless of serotype, using mild detergent treatment, buffer-exchange washing, and low-speed centrifugation. The method significantly reduces endotoxin levels while retaining up to 100% of the viral titer.
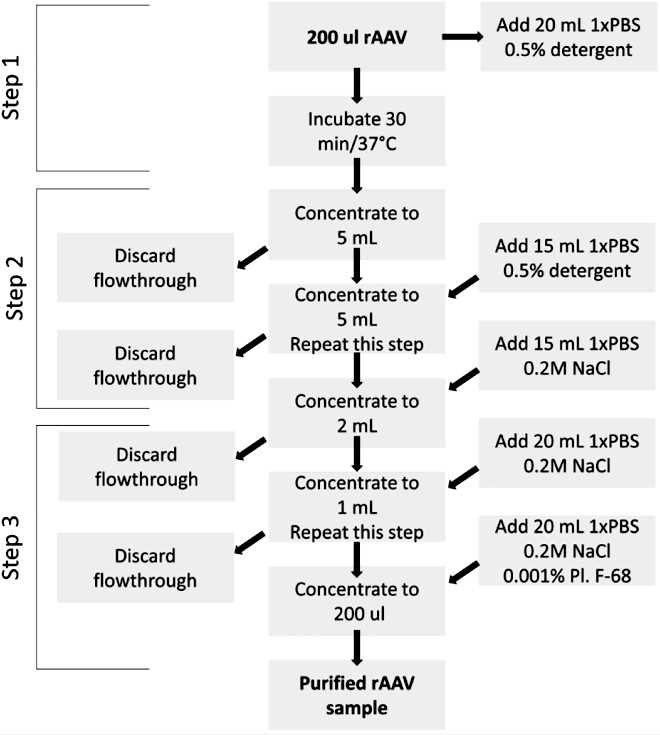 Fig.1 Decontamination of rAAV Samples. (Kondratova L, et al., 2019)
Fig.1 Decontamination of rAAV Samples. (Kondratova L, et al., 2019)
CD Formulation focuses on the problems in the development of gene therapy formulations, constantly optimizes our service system and updates our technical services, aiming to provide customers with high-quality, advanced and reliable service support. If you are interested in us, please feel free to contact us.
References
- Kondratova L, et al. Removal of Endotoxin from rAAV Samples Using a Simple Detergent-Based Protocol. Mol Ther Methods Clin Dev. 2019, 15:112-119.
Related Services

 Fig.1 Decontamination of rAAV Samples. (Kondratova L, et al., 2019)
Fig.1 Decontamination of rAAV Samples. (Kondratova L, et al., 2019)