Vaccinia Viral Vector Development Service
Inquiry
Vaccinia Virus (VACV or VV) is a linear double-stranded DNA genome of about 190 kb that can accommodate more than 30 kb of exogenous DNA. It is a large and complex enveloped virus that is widely used for high levels of cytoplasmic transgene expression and is currently widely used as a viral vector for gene therapy, due to its large genome, high transduction efficiency, and ability to carry large segments of exogenous genes. widely used as viral vectors for gene therapy. CD Formulation offers highly attenuated, host-restricted no- or low-replicative poxviral vector development services that meet a wide range of gene delivery needs in gene therapy worldwide. This technical service can lay a solid foundation for the development of novel genetically engineered live viral vectors for vaccine and biotechnology drug discovery.
Advantages of VACV Vectors Development
Vaccinia virus has many advantages such as wide host range, safety, efficient expression, and large capacity for insertion of exogenous genes, which makes it a widely used vector system after retroviral and adenoviral vectors.
- Non-integration into the host genome. VACV has a short life cycle and replicates throughout the cytoplasm, thus posing no risk of viral gene insertion into the host genome.
- Mechanism-independent replication. mRNA transcription of VACV is rarely dependent on host cell components, allowing it to adapt to different types of host cells.
- Extremely large loading capacity. VACV has a large genome that can accommodate up to 30 kb of exogenous genes.
- High titer with high infectivity . VACV can be packaged to produce high titers with good infectivity and requires only a low MOI for successful transduction.
What We Offer to Develop Vaccinia Viral Vectors?
Vector design
We first need to design the vector according to the therapeutic purpose, select the appropriate virus strain, and determine the exogenous gene sequence to be inserted.
Gene cloning and sequence optimization
The target gene is cloned into the genome of VACV and necessary sequence optimization is performed to improve the expression efficiency and stability.
Viral vector construction
Construct recombinant viral vectors by inserting exogenous genes into the genome of poxviruses using molecular biology techniques, such as restriction enzyme cleavage and ligase.
Cell culture and transfection
Select suitable host cells for culture, and transfect the constructed recombinant viral vector into the host cells to make them start to express the exogenous gene.
Virus amplification and purification
Amplify the recombinant viruses in the host cells, and then purify the viruses by physical and chemical methods to obtain high-purity viral vectors.
Quality control
The purified viral vectors are subjected to a series of quality control tests, including viral titer, purity, activity, and safety assessment.
In vitro and ex vivo validation
The efficacy and safety of viral vectors are validated in vitro cellular models and in vivo animal models to ensure their effectiveness and safety in practical applications.
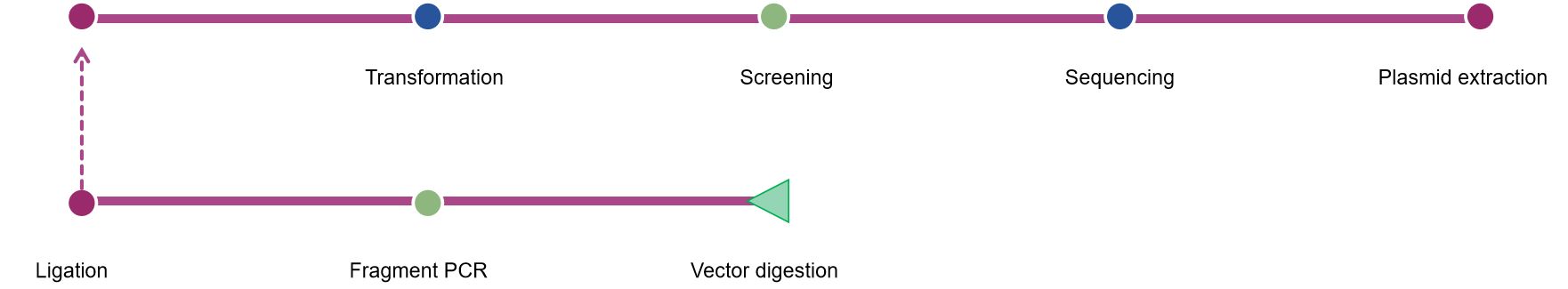 Fig.1 Our process of vaccinia viral vector construction. (CD Formulation)
Fig.1 Our process of vaccinia viral vector construction. (CD Formulation)
Application of VACV Vector Development
- Tumor therapy. In addition to directly lysing tumor cells, VACV can alter the tumor microenvironment by recruiting immune cells to cause them to secrete cytokines, thereby triggering a non-direct anti-tumor immune response. To maintain this natural benefit of VACV and enhance its safety, recombinant VACV undergoes specific genetic modifications. As an example, the attenuating properties of Pexa-Vac tumorolytic VACV are achieved by deletion of the viral TK gene, and the virus also expresses a GM-CSF transgene to enhance the host immune response. This viral vector has been designed to express various immune antigens and used in cancer therapy, including breast and colorectal cancers.
- Vaccine preparation. VACV is also quite effective as an exogenous antigen delivery system. VACV can package extremely large amounts of DNA, and these properties make VACV favorable as a potent vaccine vector against a wide range of diseases, such as against HIV and rabies.
Our Platforms for VACV Vector Development
| Technologies & Platforms |
Content Description |
| Molecular cloning technology platform |
We provide advanced and reliable molecular cloning techniques designed to insert exogenous genes into the poxvirus genome. In this process, we need to use molecular biology tools such as restriction endonucleases and DNA ligases. |
| Cell culture technology platform |
VACV needs to replicate in host cells, so cell culture is the basis for viral vector development. Equipment such as cell culture incubators, aseptic tables, and centrifuges are required. |
| Virus purification technology platform |
We have various advanced technologies including ultracentrifugation, density gradient centrifugation and chromatography for virus purification to obtain high purity viral vectors. |
| Recombinant VACV vector construction |
We have developed an advanced viral vector construction platform that allows us to construct recombinant poxviruses capable of expressing exogenous genes by inserting them into the genome of the poxviruses. The technology we offer typically utilizes non-essential gene regions of the virus as insertion sites to minimize the impact on the viral replication capacity. |
Published Data
Technology: Molecular cloning technology platform
Journal: Adv Drug Deliv Rev.
IF: 15.4
Published: 2011
Results: gene therapy, emphasizing their switch from non-replicating to replication-competent forms. Researchers discuss the use of vaccinia virus and MVA as oncolytic viruses, noting their greater immunogenicity and potential in generating T cell and B cell responses against the viral antigens and the carried transgene product.
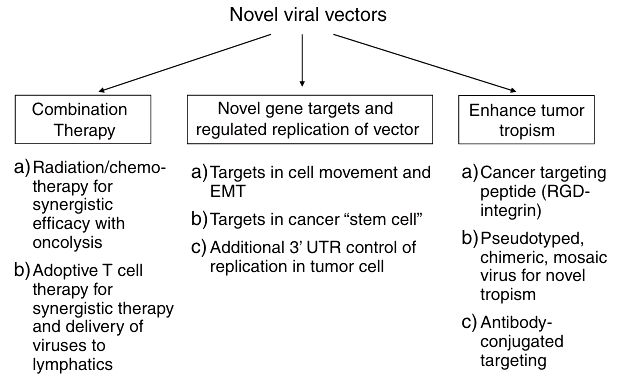 Fig.2 Approaches for improving therapeutic viral vectors. (Wu TL, et al., 2011)
Fig.2 Approaches for improving therapeutic viral vectors. (Wu TL, et al., 2011)
CD Formulation is dedicated to the development of gene therapy agents. We use our trap technology platform and skilled drug development experts to enhance the safety of VACV vectors and lower the possibility of cytopathic effects in gene therapy studies, so that researchers can more effectively aid in the advancement of vector production for gene therapy development. For your project, we provide a one-stop shop that includes designing and building appropriate vectors for gene delivery. Please don't hesitate to contact us if you have any questions or concerns.
Reference
- Wu TL, Zhou D,et al. Viral delivery for gene therapy against cell movement in cancer. Adv Drug Deliv Rev. 2011, 63(8):671-7.
Related Services

 Fig.1 Our process of vaccinia viral vector construction. (CD Formulation)
Fig.1 Our process of vaccinia viral vector construction. (CD Formulation) Fig.2 Approaches for improving therapeutic viral vectors. (Wu TL, et al., 2011)
Fig.2 Approaches for improving therapeutic viral vectors. (Wu TL, et al., 2011)