Gene Therapy Residuals Testing for Bioprocess Validation
Inquiry
Process-related impurities are introduced at various stages of the manufacturing process for gene therapy products. These impurities may arise from upstream steps such as cell and culture growth or harvesting, downstream processes, etc. These impurities may adversely affect the gene therapy end product, thereby affecting critical quality parameters and the safety of the gene therapy. Therefore detection and quantification of these impurities and demonstration of effective removal of these impurities is critical to validate the manufacturing process.
Residual testing for bioprocess validation plays a key role in ensuring the safety of gene therapy products. Common residue testing methods include nucleic acid detection (e.g. PCR), and chromatography (e.g. HPLC, GC-MS). Trace amounts of residual substances can be quantitatively detected by these methods. CD Formulation has advanced testing equipment and professional laboratory personnel, which can provide comprehensive quality control support for gene therapy research. We can help researchers to obtain reliable test results and provide strong support for gene therapy research.
Importance of Gene Therapy Residual Testing for Bioprocess Validation
- Safety assurance. Residual host cell DNA, plasmid DNA, or other biological material may contain sequences from viruses, bacteria, or other pathogens that may cause an infection or immune response in the patient.
- Reduced risk of carcinogenesis. Host cell DNA may contain oncogenic genes that, if activated in the patient, may increase the patient's risk of cancer.
- Prevent immunogenicity problems. DNA of microbial origin contains certain structures that may trigger an immune response in the body, resulting in reduced therapeutic efficacy or adverse reactions.
- Ensure product quality. The presence of residues may affect the purity and potency of the gene therapy product, thereby affecting the efficacy of the treatment and patient safety.

Our Gene Therapy Residual Testing for Bioprocess Validation
Host cell DNA residue testing
Some cell lines are commonly used in the production of viral vectors and therefore require testing of residual DNA from these cells. We can accurately, sensitively and consistently quantify residual DNA and control the amount of process-related impurities, such as host cell DNA, at an acceptable level.
Risk component residue testing
In gene therapy, there is a need to accurately quantify specific transforming sequences with safety risks that may remain. These risk elements may originate from the cell lines used in the production process.
Plasmid DNA residue testing
During the production of plasmid vectors, plasmid DNA residues need to be tested. We can quantify plasmid DNA residues with high sensitivity, consistency and specificity.
Enzyme residue testing
During the production of gene therapy products, substances such as ectopic nucleases and protein residues need to be monitored and analyzed to ensure the quality and safety of the final product.
Our Gene Therapy Residual Testing Instruments for Bioprocess Validation
qPCR
Plate readers
Mass spectrometers
Chromatography equipment
Detectors associated with chromatography equipment
- Corona Charged Aerosol (CAD)
- Evaporative Light Scattering (ELSD)
- Fluorescence (FL)
- Photodiode Array (PDA)
- Refractive Index (RI)
- Ultraviolet (UV)
- Electrochemical (ECD)
- Flame Ionization Detector (FID)
- Thermal Conductivity Detector (TCD)
Highlights of Our Gene Therapy Residual Testing for Bioprocess Validation
- We have the experience to save research time and minimize costs.
- We have a wide range of instruments dedicated to the detection of process-relevant impurities in samples.
- We can support you at every stage of the gene therapy development process with reliable technologies, tools, solutions and scientific experience.
- We comply with strict regulations and ensure high-quality data.
Published Data
Technology: Quantification of residual host cell DNA in rAAV gene therapy products
Journal: Mol Ther Methods Clin Dev
IF: 9.9
Published: 2019
The article discusses the use of recombinant adeno-associated virus (rAAV) in gene therapy and the importance of monitoring host cell DNA contamination. Traditional quantification methods for residual DNA in rAAV involved a proteinase digestion step before qPCR analysis. However, the necessity of this step was in question. The study aimed to simplify the process and found that proteinase digestion was not required for DNA release from rAAV. Instead, the addition of Tween 20 was crucial for accurate DNA quantification. As a result, a new, streamlined method was developed that only requires the addition of Tween 20 to the sample. This method has been validated with rAAV9 and other serotypes, offering a faster and more automated approach for high-throughput applications.
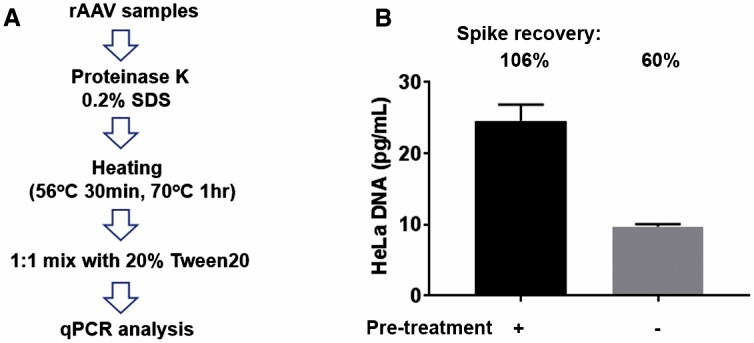 Fig.2 Evaluation of the existing residual DNA assay with proteinase K digestion. (Wang Y, et al., 2019)
Fig.2 Evaluation of the existing residual DNA assay with proteinase K digestion. (Wang Y, et al., 2019)
Gene therapy formulation development is an important part of gene therapy research. CD Formulation, based on years of experience in viral vector preparation and quality control, provides customers with a full range of vector characterization and process residue testing services, which provide reliable quality assurance for the rapid filing of gene therapy products as well as subsequent large-scale production. If you are interested in us, please feel free to contact us.
References
- Wang Y, et al. A Digestion-free Method for Quantification of Residual Host Cell DNA in rAAV Gene Therapy Products. Mol Ther Methods Clin Dev. 2019 May 18;13:526-531.
Related Services


 Fig.2 Evaluation of the existing residual DNA assay with proteinase K digestion. (Wang Y, et al., 2019)
Fig.2 Evaluation of the existing residual DNA assay with proteinase K digestion. (Wang Y, et al., 2019)