Gene Therapy Formulation Process-Related Impurities Testing
Inquiry
A range of residual materials, including process-related and product-related contaminants, may be produced during the manufacturing of gene therapy formulations. Raw materials, reagents, host residual DNA, and other viral particles utilized in the production process are among them, if they are not entirely eliminated, they may have a detrimental effect on the quality and purity of the gene therapy formulation.
CD Formulations offers comprehensive and reliable process-related impurity testing to ensure the purity and safety of gene therapy formulations. This important process not only helps researchers meet regulatory standards but also ensures the safety and efficacy of their products.
Importance for Gene Therapy Formulation Process-Related Impurities Testing
- Ensure product safety and efficacy. The presence of impurities may affect the safety and efficacy of gene therapy formulations, so detection of these impurities is critical.
- Reduce immunogenicity. For example, empty capsid viruses are an unwanted source of potential antigenic material that may induce or enhance capsid-triggered anti-AAV innate and adaptive immune responses.
- Ensure product quality. To ensure consistency and quality of gene therapy formulations, production process impurity testing is required to ensure that adverse side effects are minimized.
- Compliance with relevant requirements. Detection of impurities in the gene therapy formulation development process is necessary to meet the requirements of the relevant regulatory agencies.
- Optimize the process. By detecting impurities, the production process can be identified and optimized to reduce impurities and improve the overall quality of the gene therapy formulation.
Explore Our Gene Therapy Formulation Process-Related Impurities Testing
In developing gene therapy formulations, process-related impurity testing is a critical component to ensure product safety and efficacy. These impurities may originate from the manufacturing process, including cellular matrices (e.g. residual host cell DNA, host cell proteins), components and additives of cell culture and fermentation media, and impurities from downstream process sources.
We provide comprehensive and accurate impurity testing services related to the gene therapy agent development process. This service support not only has high-quality quality assurance but also can provide customized and personalized solutions according to the characteristics and needs of the customer's project, to maximize the help of the customer to improve the accuracy of the test and obtain the most reliable test results.
In the selection of testing technology, we will provide the most suitable testing solutions for different impurity tests after comprehensive analysis, for example, for residual host cell DNA, we can provide DNA hybridization, quantitative PCR, and other detection methods, for residual host cell protein and protein residues in the cell culture medium, we can be measured by ELISA, SDS-PAGE and other methods. The development of gene therapy formulations provides alternative solutions for the treatment of various diseases, but to ensure the safety and reliability of gene therapy agents and to reduce the emergence of side effects requires the continuous optimization of the process-related impurity detection technology, the pursuit of a more accurate and wider range of detection of a variety of possible impurities, to ensure that the impurities in the agents developed by us are in a safe and reasonable range to further ensure the safety of the gene therapy agents. Ensure the safety of gene therapy formulations.
We customize gene therapy formulation process-related impurities testing
Our Technologies for Gene Therapy Formulation Process-Related Impurities Testing
| Platforms & Technologies |
Content Description |
| DNA hybridization |
DNA hybridization is the process of joining two single-stranded DNAs to form a double-stranded DNA. DNA hybridization can be used to assess the genetic similarity between two DNA populations or to detect specific DNA sequences in a population of DNA molecules. With DNA hybridization, residual host cell DNA can be effectively detected by hybridizing a labeled probe with the sample DNA. |
| Fluorescent staining |
We utilize specific fluorescent dyes that bind to double-stranded DNA. The fluorescence intensity is proportional to the concentration of DNA and is commonly used to quantify the concentration of DNA. The fluorescent staining method has shown to be convenient and fast in practical applications, especially in the detection of DNA contamination of RNA virus vectors. |
| Quantitative PCR (qPCR) |
This is a PCR-based quantitative method for detecting specific DNA from a defined source and amplifying it with a specific gene sequence. qPCR technology plays an important role in ensuring the safety and efficacy of gene therapy products, and is especially used for the detection and quantification of residual host cell DNA. |
| Enzyme-linked immunosorbent assay (ELISA) |
ELISA can be effectively and reliably applied to detect protein residues in host cell proteins or cell culture media, and shows simplicity and high sensitivity. With the use of particular antibodies, we measure particular proteins in samples by observing the color shift that results from the antigen-antibody response. |
Highlights of Our Gene Therapy Formulation Process-Related Impurities Testing
- We provide comprehensive process-related impurity testing services for gene therapy formulation development to help researchers ensure that gene therapy formulations being developed are safe and comply with relevant agency regulatory requirements.
- We can provide process-related impurity testing solutions that are customized to the specific needs of each client's project to maximize the development and research of gene therapy agents.
- We can accurately detect and quantify a wide range of contaminating impurities, including but not limited to plasmid DNA, host DNA residues, host RNA residues, etc., that are present during the development and manufacture of gene therapy agents.
- We offer one-stop, high-quality impurity testing services and provide a detailed validation report to confirm compliance at the end of the testing.
Published Data
Journal: Biomedicines
IF: 4.7
Published: 2014
This article focuses on AAV product-related impurities described in carriers prepared for clinical development. Current challenges in the clinical development of AAV-based test products are the identification, characterization, and batch-to-batch control of process- and product-related impurities, which are present even in highly purified formulations. Particularly challenging are AAV carrier product-related impurities, which are very similar to the carriers themselves, and determining acceptable levels of these impurities in carriers prepared for human clinical product development requires careful risk and feasibility assessments to obtain new product licenses.
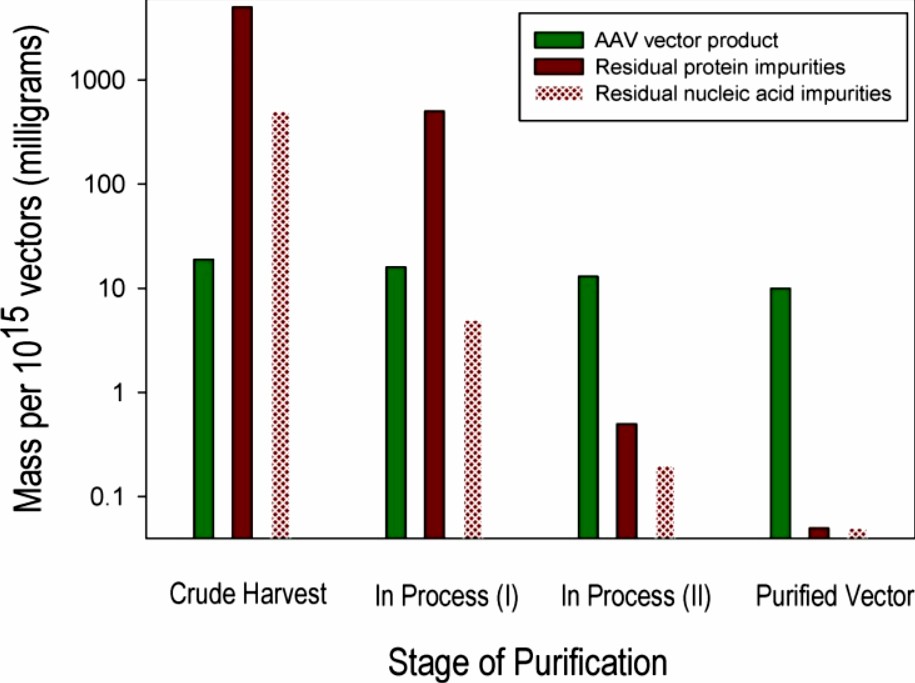 Fig.1 Adeno-associated virus (AAV) vector and impurity levels during purification. (Wright JF. 2014)
Fig.1 Adeno-associated virus (AAV) vector and impurity levels during purification. (Wright JF. 2014)
CD Formulations has many years of experience in gene therapy formulation development. Relying on our advanced technology platform and professional service support, we can maximize your research results in gene therapy formulation development. If you are interested in us, please feel free to contact us.
References
- Wright JF. Product-Related Impurities in Clinical-Grade Recombinant AAV Vectors: Characterization and Risk Assessment. Biomedicines. 2014, 2(1):80-97.
Related Services

 Fig.1 Adeno-associated virus (AAV) vector and impurity levels during purification. (Wright JF. 2014)
Fig.1 Adeno-associated virus (AAV) vector and impurity levels during purification. (Wright JF. 2014)